
When certain compounds in a solution remain in the solution while others pass through a membrane, it is called a membrane separation. Another function of the membrane is to change the solution’s composition. This depends on the respective permeability rates. The capacity of a membrane to either completely block, significantly reduce, or significantly increase the rate of permeation is one indicator of its performance.
How does membrane separation work?
The pressure difference that exists across a membrane is what allows for membrane separation. One side of the membrane goes through high pressure. This leads to the passage of smaller molecules through the pores while preventing larger molecules from passing through.
Many factors, including concentration, pH, temperature, and the size of the separated molecules, determine the precise method of membrane separation.
4 types of membrane filtration?
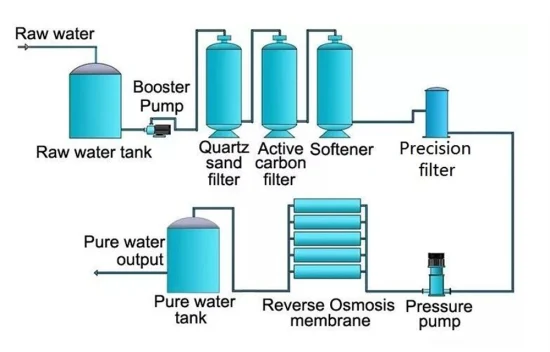
Ultrafiltration, reverse osmosis (RO), microfiltration (MF), and nanofiltration NF) are all kinds of membrane separation.
- Microfiltration.
To remove microbes and microscopic particles from a gas or liquid, microfiltration (MF) uses a tiny pore membrane.
The gas or liquid goes through the membrane by applying pressure. Additionally, any particles that are too big to fit through the pores remain on the membrane’s surface. Because of this, separation occurs on bigger particles like suspended solids, algae, and bacteria.
- Ultrafiltration.
UF differs from MF in that it uses a smaller pore membrane. The separation of bigger molecules, such as proteins, takes place by applying pressure to the gas or liquid. This causes it to pass through the membrane.
There can be purification or concentration of substances via ultrafiltration, depending on the specific purpose.
- Nanofiltration.
Membranes used in NF have pores that are even smaller than those in UF. The gas or liquid is forced through the filtering membrane by applying pressure. This separates smaller molecules, such as salts. Industrial water undergoes nanofiltration to eliminate minerals and salts that may alter its quality and taste.
Nanofiltration can work well in the chemical and pharmaceutical industries. It will help to isolate particular ions and other tiny molecules, as well as in the beverage and food industries, for purifying liquids. NF is a powerful method for precisely filtering liquids due to its high selectivity.
- Reverse osmosis.
The process of RO involves separating water and dissolved compounds using a semi-permeable membrane that has extremely tiny pores. The technique relies on osmosis, in which water flows in the opposite direction of its regular flow. This is achievable by adding high pressure to the water.
The pressure forces dissolved substances, such as minerals and salts, out of the cell membrane. Because it can filter out pollutants and dissolved impurities, RO is great for industrial water treatment.
Many other businesses rely on reverse osmosis to purge water of certain small molecules and ions. This includes those dealing with pharmaceuticals, drinks, and food. One of reverse osmosis’s most notable features is the water purity it achieves with such remarkable efficiency.
Is membrane filtration effective?
Since membrane pretreatment systems often use fewer chemicals and less space than traditional pretreatment systems, membrane separation is popular as a more effective pretreatment than the traditional one.
Things that a membrane filter gets rid of
One common separation method in water purification systems is membrane filtration. In addition to removing contaminants, including viruses, total suspended solids, silt, and bacteria, membrane filters can also reduce turbidity and the growth of viruses.
Does membrane filtration remove bacteria?
By removing viruses, bacteria, and other pathogens from water, water filter membranes can treat drinking water as well as pool and spa surroundings. This helps to prevent and control diseases. The food and drink sector is another potential user for its use in liquid purification and concentration.
The advantages and disadvantages of membrane processes?
Comparing membrane processes to other methods of separation, there are a variety of advantages and disadvantages involved.
The advantages include:
- Since the membrane method calls for the use of mostly harmless and easy-to-produce ingredients, it may be better for the environment than other procedures.
- With the exception of pervaporation, most membrane processes can separate without a phase shift. Unless there’s a need for a substantial amount of energy to raise the feed stream pressure to force the permeating component(s) through the membrane, the energy requirements will be minimal.
- A wide range of separation selectivities is achievable due to the membranes’ adaptability to a wide variety of polymers and inorganic media.
- A very simple flowsheet is essentially what membrane processes show. Unlike many other processes, this one doesn’t include complex control schemes, auxiliary equipment, or moving parts (with the exception of compressors and pumps). They are able to provide a low-maintenance procedure because of how simple it is to operate.
- Without using a lot of energy, membrane processes can extract small but valuable components from a stream.
- Membrane processes may meet a great deal of separation requirements. This is because they can separate at scales ranging from the molecular to the observable particle level.
- It is possible to create membranes with very high selectivities for the separation of components. Typical values for relative volatility in distillation processes are significantly lower than these selectivities.
Disadvantages
- Poor separation performance could be the consequence of poorly managed membrane manufacturing. This can lead to membranes with a large pore size distribution.
- Equipment costs can be quite expensive.
- Using cross-flow feed at high flow rates might harm materials that are sensitive to shear.
- Membrane fouling effects, which reduce permeate flux, are common in these processes. It might be necessary to implement costly regeneration and cleaning schemes.
Is membrane filtration expensive?
Membrane filtration is typically more cost-effective than other technologies. Its fewer processing steps, lower energy costs, and easier installation are the reasons behind this. Concurrently, it increases both the purity and the total yield.
 With membrane separation, you can get more done in less time while still getting a higher yield and more purity. Furthermore, there are no expenses linked to the removal and disposal of this residue when using membrane filtration. This is because it does not produce a filter cake.
With membrane separation, you can get more done in less time while still getting a higher yield and more purity. Furthermore, there are no expenses linked to the removal and disposal of this residue when using membrane filtration. This is because it does not produce a filter cake.
Factors Affecting Membrane Separation
pH levels.
The stability, charge, and solubility of the separated compounds can influence the rate of diffusion across the pores of the membrane. The pH level of the substances affects everyone.
Changes in pH can affect a material’s solubility and, consequently, its permeability to holes. Another factor that can influence the stability and structure of the membrane material. This, in turn, affects its ability to operate efficiently over time. The pH, concentration, pressure, and temperature levels of the membrane separation system can be controlled to produce water of excellent quality.
Temperature.
How quickly things diffuse through a membrane’s pores depends on a number of physical qualities, some of which are temperature-dependent. These properties include solubility and viscosity.
Substances may become more permeable to pores when their viscosity decreases as their temperature rises. A drop in temperature can produce the same result in various contexts. Temperature can also have an impact on the stability of the membrane material, which determines how well it keeps its shape and function over time. Membrane separation procedures are also susceptible to temperature-induced changes in filtration and pumping.
Concentration.
Crucial to the membrane separation process, concentration affects the efficiency of separation. The separation pressure and pore size are both affected by the concentration of the substances being separated. The pressure needed to push a substance through pores grows in relation to its concentration. The process’s effectiveness can drop if something clogs the membrane.
Sometimes, it is possible to create a situation where the chemical’s concentration is higher on one membrane’s side than the other. This refers to a concentration gradient. This contributes to the divide. The concentration of the material can affect the rate of diffusion through the pores. This, in turn, affects the separation’s effectiveness.
Pressure.
The process of membrane separation is dependent on pressure. Depending on the pressure difference across a membrane, substances can either pass through it or not.
The efficiency of the membrane separation process is often proportional to the pressure. However, the characteristics and size of the particles being separated, as well as the durability and strength of the membrane material, place limits on that pressure.
The membrane separation process can be more consistent and effective with the help of a pump by keeping the pressure difference across the membrane at the correct level.
Conclusion
If you need to treat or purify water, membrane filtration is a great and flexible option. Its accuracy, adaptability, and low chemical consumption make it a priceless asset in the fight for water safety.
The use of membrane separation is important in improving the sustainability and quality of water in various applications, such as industrial processes, wastewater reclamation, and drinking water treatment. Among the several advantages of the membrane separation method are its decreased manufacturing costs, decreased energy consumption, adaptability, pathogen elimination rates of up to 100 percent, and ability to sterilize heat-sensitive materials.


 Alkaline Cleaning:
Alkaline Cleaning:


 Post-filtration:
Post-filtration:

 The difference between UF and NF filtration?
The difference between UF and NF filtration?
 Distillation by Evaporation
Distillation by Evaporation