
A dosing metering pump is equipment for precisely dosing one fluid into another. Typically, dosing metering pumps are used in businesses that place a premium on the exact addition of substances or chemicals to larger quantities, such as pharmaceutical production.
When buying a dosing metering pump, it’s important to consider the following factors: wetted path material types, discharge pressure, liquid viscosity, flow rates, and temperature. A metering pump’s fundamental components are its motor and pump head. You can adjust them electronically or manually.
Metering pumps frequently use piston-driven positive displacement pumps to measure fluid in the reservoir chamber. Pistons operate these pumps, and different designs can be tailored to specific needs.
Type of pump commonly used for metering and dosing applications
A dosing metering pump can be either positive displacement or centrifugal. When it comes to accuracy, though, positive displacement pumps outperform centrifugal pumps.
Here are some of the most popular dosing metering pumps:
Piston/Plunger
This device comprises a housing that houses the component. During the suction stroke, fluid enters the chamber as the piston reciprocates within the housing. Afterward, it is pushed out during the discharge stroke. You can precisely obtain accurate information regarding the quantity of fluid being dosed into the fluid stream. This is done by knowing the piston’s position and the chamber’s volume.
Due to this design, piston/plunger types have the most precise fluid metering capabilities among many dosing metering pumps. But this also means the fluid stream doesn’t get any fluid during the suction stroke. Additionally, the pump allows for discharge in pulses. Because of these features, these types of pumps aren’t good choices for many uses. Wear and tear, caused by the piston rubbing against the cylinder, further reduces the pump’s lifetime.
Plunger Pump
Plunger-metering pumps use plungers to reciprocate. It is a forward-stroke, single-acting pump that releases fluids. To drain the fluid, they feature a suction check valve. A discharge check valve drives the fluid through as the plunger moves forward.
When it comes to high-pressure applications, plunger metering pumps are superior to piston metering pumps due to their design. Compared to piston metering pumps, they are faster and can handle pressures of more than 50,000 psi.
Gear Pump
This pump type mimics the action of a peristaltic metering pump but eliminates the tube. The fluid flows into or gets sucked into a vacuum created by the gear’s intermeshing motion. The gears cause the fluid to travel in a revolving motion from the inlet to the outlet valve.
Because their construction can handle high pressure, gear metering pumps are frequently utilized with fluids with a high viscosity. Applications that include hydraulic fluid power are the ones that make use of these pumps the most often.
Syringe Metering Pumps
Withdrawal and infusion syringe metering pumps are the two main types. Both have one primary function: to transport a set volume of fluid. Infusion types allow for the controlled administration of small fluid volumes at set intervals and pressures. Pharmaceutical and medical testing fluid samples are automatically withdrawn with withdrawal syringe metering pumps.
Dosage administration is the primary function of these pumps. They are subject to strict guidelines for both flow rate and speed.
To infuse or withdraw fluid, this pump uses a piston. They are unsuitable for automated applications due to their slow operation. When there are a lot of syringe-metering pumps in a system, the pressure variations can be devastating. Small amounts of fluid can only be moved by the reservoirs of syringe metering pumps.
Peristaltic Metering Pump
These pumps differ in design from their diaphragm-driven and piston-driven counterparts. The medium passes through a tubing or hose by peristaltic metering pumps. Rollers mounted on a rotor finish the fluid control mechanism. The tubing draws in the fluid flow by creating negative pressure. When the tubing is filled, the rotor’s rotation stops the flow. The fluid in the tubing flows in the direction of pumping through the rotor’s revolving action.
The roller releases tension from the tube, and the hose aligns with the flow as it finishes its motion. The subsequent delivery of the medium might fill the tubing since negative pressure is once again generated.
Mechanical Diaphragm
This pump type uses a mechanical diaphragm instead of a piston. The diaphragm draws liquid into the chamber during expansion and injects it into the fluid flow during compression. Eccentric cams drive the diaphragms by converting the motor’s rotating motion into reciprocating motion.
The amount of fluid sucked and injected into the fluid stream can be accurately measured by observing the expansion and contraction levels, just like with piston pumps. Mechanical diaphragms are ideal for dosing poisonous fluids. This is because of their tightly sealed construction, which is another advantage. They also don’t wear out as quickly as piston pumps do.
Pulsed discharge is just one of the impacts that can harm the mechanical diaphragm. Nevertheless, a two-diaphragm setup can lessen the impact of such effects.
 Hydraulic Diaphragm
Hydraulic Diaphragm
A hydraulic diaphragm is similar to a mechanical diaphragm but powered by hydraulics. Changing the hydraulic fluid’s pressure is one way to expand or contract the diaphragm.
The pulsed discharge is eliminated by hydraulic double-diaphragm motors using two diaphragms. Furthermore, the even load distribution on hydraulic diaphragms makes them more durable than mechanically driven ones. This allows them to handle loads that mechanical ones can’t match.
Applications of a dosing metering pump
The water treatment, food, and pharmaceutical industries are just a few of the many that use a dosing metering pump. Injecting precisely measured amounts of the substance into the fluid stream is essential in several industries, and a dosing metering pump is an ideal solution for this.
Factors to consider when selecting a dosing metering pump include:
- Operating pressure
- The necessary degree of precision
- The injectable fluid
How do you control the flow of a dosing pump?
There are a number of methods for controlling a dosing metering flow rate. This is the quantity of liquid given per unit of time to guarantee consistent and precise dosing.
Use a programmable controller
You can schedule the dosing and adjust the flow rate using some dosing pumps‘ programmable controllers. Precise chemical dosing at predetermined intervals can be an easy method to regulate the flow rate.
Control the stroke rate
A dosing pump’s stroke rate is the number of cycles it completes in one second. One way to regulate the flow rate is to alter the stroke rate, which affects the number of pump cycles.
Control the diameter of the orifices
You can adjust the dosing pump’s flow rate by adjusting the diameter of its orifices. Less liquid can move through an opening in the same period of time when its size is smaller.
Control the inlet pressure
The inlet pressure of a dosing metering pump is the pressure of the liquid entering the pump. A change in the inlet pressure causes a corresponding change in the liquid’s pressure entering the pump. This, in turn, controls the flow rate.
Use a flow meter
One way to measure how fast liquids are moving through a system is with a flow meter. To make sure the right amount of chemical distribution, attach a flow meter to the dosing metering pump and adjust the flow rate.
Control the stroke length
The stroke length of a dosing pump is the distance it travels in one cycle. You may regulate the flow rate and the quantity of liquid dispensed per cycle by adjusting the stroke length.
Control the outlet pressure
The liquid’s pressure at the point where the dosing pump is removed from the system is known as the outlet pressure. You may change the flow rate by adjusting the liquid’s pressure leaving the pump. Outlet pressure adjustment is the method for doing this.
Conclusion
Due to the fact that different applications call for varied degrees of precision and flow, the definition of dosing changes across all sectors. Consequently, we supply a variety of dosing pumps that support a wide range of flow rates and degrees of precision.
You can get reliable and inexpensive replacements for the old-fashioned, conventional gear pumps with the help of an excellent metering pump. In both high-pressure and low-flow applications, it provides continuous dosing methods. The dosing-metering pump is ideal for both continuous and sporadic tasks. This is because they guarantee smooth pumping with minimal shear and no pulsation.
Regarding industrial dosing metering pumps, our team at Express Drainage Solutions guarantees reliable, long-lasting, and top-notch technology. We provide the top specialists in the industry to help our customers install and maintain these pumps so their industrial production runs smoothly.




 Working conditions
Working conditions
 When applied to metal surfaces, hydrogen peroxide can aid in the breakdown of organic deposits. This allows other CIP chemicals to access and dissolve them more easily. When compared to other CIP chemicals, hydrogen peroxide often needs less time in contact with the metal surface while still effectively penetrating deep deposits. During inactivity, it shields against corrosion and effectively stops the deposition of new sediments.
When applied to metal surfaces, hydrogen peroxide can aid in the breakdown of organic deposits. This allows other CIP chemicals to access and dissolve them more easily. When compared to other CIP chemicals, hydrogen peroxide often needs less time in contact with the metal surface while still effectively penetrating deep deposits. During inactivity, it shields against corrosion and effectively stops the deposition of new sediments.
 The solution is returned to the tank for reuse after cleaning the line thoroughly. Last but not least, the line is supplied with fresh rinse water. Purge the tanks and pipes of the cleaning solutions used in the previous process; that is the goal of this operation. Collecting and storing rinse water for subsequent use in washing machines is a common practice. It is possible to heat the water and cleaning products to your liking.
The solution is returned to the tank for reuse after cleaning the line thoroughly. Last but not least, the line is supplied with fresh rinse water. Purge the tanks and pipes of the cleaning solutions used in the previous process; that is the goal of this operation. Collecting and storing rinse water for subsequent use in washing machines is a common practice. It is possible to heat the water and cleaning products to your liking.
 Your plumbing system will experience a hammer if the circuit pressure drops and this water turns into steam. Another reason pipes don’t last as long as they should is that carbonic forms during the unintended phase between shifts of condensate and steam. This acid damages the pipe wall.
Your plumbing system will experience a hammer if the circuit pressure drops and this water turns into steam. Another reason pipes don’t last as long as they should is that carbonic forms during the unintended phase between shifts of condensate and steam. This acid damages the pipe wall.
 Forward osmosis
Forward osmosis 

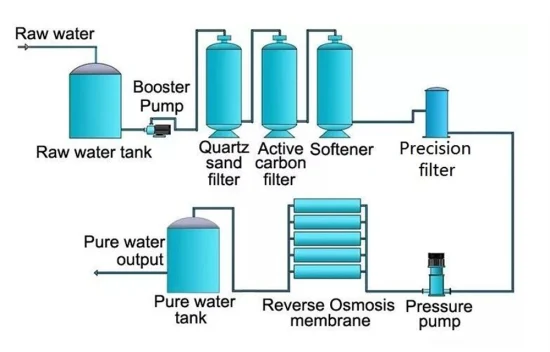 Cleaning using chemicals
Cleaning using chemicals